CAR-T
Research & Innovation
CAR-T
CAR-T
Cancer has long been one of the leading causes of death not only in Taiwan but also worldwide. Cell therapy is a developing approach for treating cancer and other diseases that involves the administration of specific cells, such as immune cells or stem cells. Current CAR-T cell therapy have shown remarkable clinical responses in hematological malignancies. However, solid tumors (such as lung cancer, breast cancer, and colorectal cancer, etc.) are still regarded as the most difficult field to conquer, despite accounting for more than 90% of all cancer patients. Therefore, it is necessary to develop new strategies to improve CAR-T cell therapy to be more effective against solid tumors. Professor Cho Der-Yang, the Superintendent of the China Medical University Hospital, and the Director of the Translational Cell Therapy Center, led the research team and has achieved a significant global breakthrough. Preclinical studies have yielded remarkable results after years of research and development of “allotransplantation, non-viral driven multi-target nanobody-based CAR.BiTE-gdT cell therapy”, including patents, publication on prestigious academic journal “Advanced Science”, and the most remarkable was the Investigational New Drug (IND) Application approved by U.S. FDA and undergoing Phase I/II clinical trials in Taiwan. We anticipate that the innovative allogeneic CAR-T cell therapy will yield significant outcomes in the future.
Main Product
Allogeneic Multi-Targeting Nanobody-Based CAR.BiTE-GDT (CAR001)
Product Introduction
CAR.BiTE-GDT has the potential to fight against a variety of refractory solid cancers.
“Off-the-shelf”, allogeneic transplantable, capable to solve the risk that cancer patients spend time waiting for the manufacture of autologous cell product.
Non-viral gene-modified strategy prevent the risk of mutation in cell product.
Allogeneic cell products can be prepared in large quantities and standardized, and lessen production costs which is expected to be reduced to about under 1/5 compared with autologous CAR-T.
Can be applied to patients with HLA-G-expressing cancers, and can be combined with targeted therapy and chemotherapy to prolong survival time.
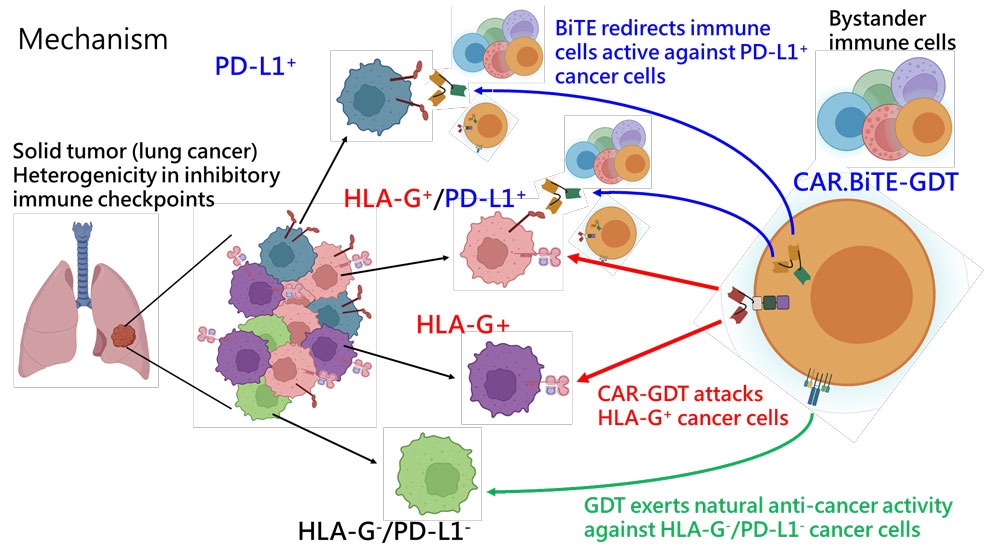
CAR001 is designed to overcome immune-suppressive microenvironment and antigen dilemma of solid tumors
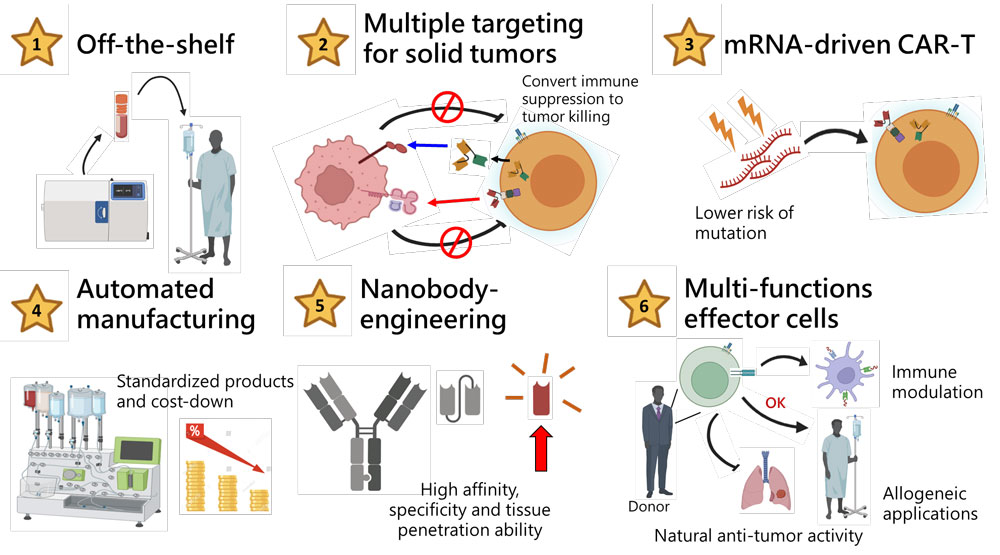
Highlights of CAR001
Achievement 1
The Investigational New Drug (IND) Application approved by U.S. FDA and TFDA, and undergoing Phase I/II clinical trials in Taiwan (7th patients now).
Achievement 2

Taiwan Patent NO. I823402

2023 Tech Innovation Excellence Award (TIE Award)
.jpg)
Silver Medal, 21th Taipei Biotech Awards (2024)
Contact Information
Translational Cell Therapy Center, seso4505@gmail.com. ext. 12406, Shi-Wei Huang, Associate Research Fellow
