Exosome
SOA101
SOA101 (Nanobody-based trispecific T cell engager, Nb-TriTE)
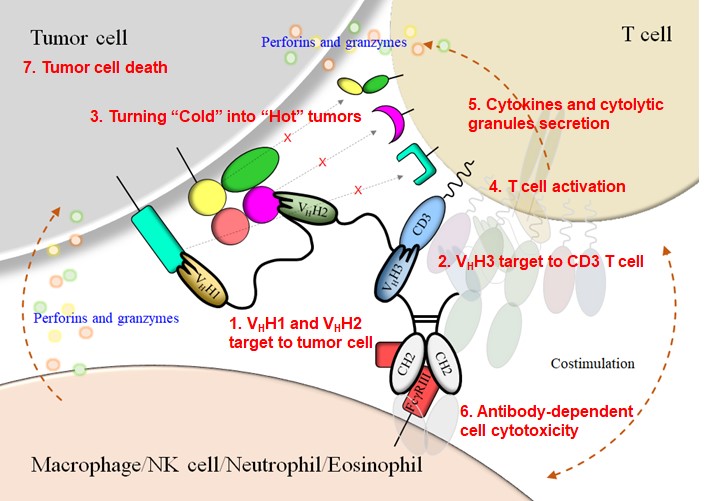
SOA101 was developed as a nanobody-based trispecific T cell engager (Nb-TriTE), capable of simultaneously binding to T cells, macrophages, and cancer cells while redirecting T cells towards tumor cells expressing PD-L1- and/or HLA-G. Nb-TriTE showed broad spectrum anti-tumor effects in vitro by augmenting cytotoxicity mediated by human peripheral blood mononuclear cells (PBMCs). In a humanized murine non-small cell lung cancer (NSCLC) model, Nb-TriTE exhibited superior anti-cancer potency compared to monoclonal antibodies and bispecific T cell engagers (BiTE). Nb-TriTE at the dose with pharmacoactivity did not induce additional enhancement of circulating cytokines secretion.
Product Introduction
SOA101 targets two ICPs (HLA-G and PD-L1)on tumor cell surface, and another nanobody moiety recruits T cells active against the tumor cells
Highlights
▪ Targets two anti-tumor ICPs on tumor cells, and used for T cell redirecting
▪ The nanobodies are connected and fused with human IgG1 Fc domain
▪ Fc domain is designed for prolonging the half-life of Nb-TriTE
▪ Enhances the integrity of tumor cells and T cells
▪ Conquers the immune-suppressive cold- to hot-tumor microenvironment
▪ Triggers T cell proliferation and activation
▪ Facilitates antibody-dependent cell cytotoxicity
Achievement1
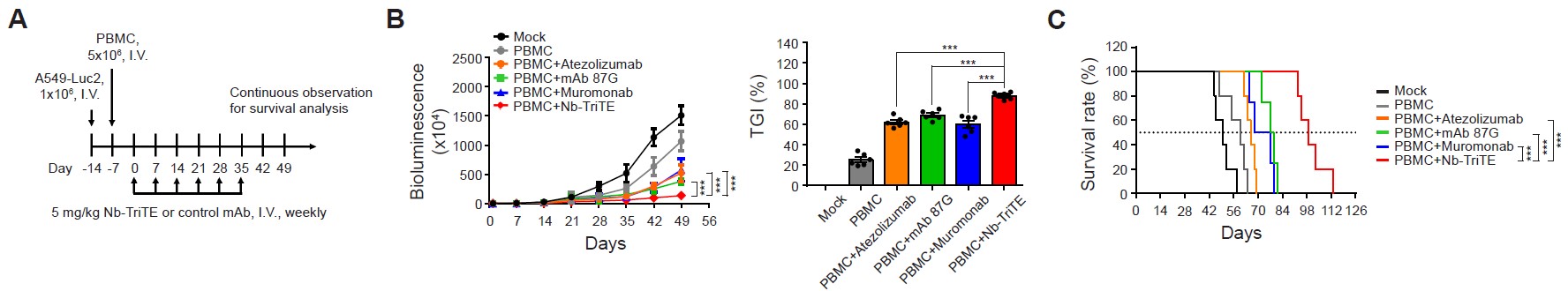
Nb-TriTE suppresses NSCLC tumor growth and extends survival in humanised xenograft mice. (A) Treatment protocols for animal studies comparing the anti-cancer efficacy of Nb-TriTE and commercial monoclonal antibodies. (B) Tumor size was monitored and recorded weekly using the representative in vivo imaging system (IVIS). Tumor changes were quantified as growth inhibition (TGI). (C) The survival rate of mice was plotted using the Kaplan–Meier method. ***p<0.001.
Achievement2
The Investigational New Drug (IND) Application approved by U.S. FDA for phase I/II clinical trials (PIND: 169847), and undergoing IND submission in Taiwan
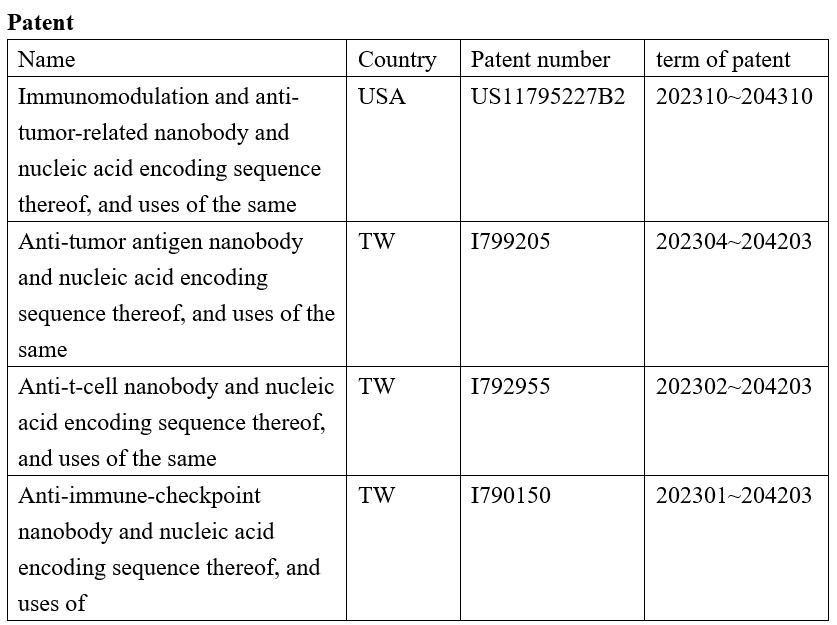
U. S. Patent of SOA101
%e8%81%96%e5%ae%89%e7%94%9f%e9%86%ab%e9%9b%bb%e5%ad%90%e8%ad%89%e6%9b%b8PatCntr_eGrantPDF_pages-to-jpg-0003(1).jpg)
Taiwan Patent of HLA-G nanobody
.jpg)
Taiwan Patent of PD-L1 nanobody
.jpg)
Taiwan Patent of CD3 nanobody
(1).jpg)

Award
The 19th National Innovation Award in the Enterprise Innovation Category, 2022. (Breaking through the dilemma of tumor microenvironment with an innovative tri-specific nanobody based T-cell engager)
Contact Information
Unit/Telephone (including extension)/E-Mail
Barry Yu-Chuan Lin/Translational Cell Therapy Center/+886-4-2205-2121, ext.12409/xup6m4fm06@gmail.com
